As a machine manufacturer, we want to make sure our product is installed, connected, and set-up correctly for optimum performance.
Our qualified technicians and engineers ensure fast and efficient equipment commissioning from unpacking to training. We assist in the validation process and provide installation and operational qualification (IQ/OQ) and comprehensive documentation packages based on requirements.
REPAIR AND MAINTANENCE
For scheduled maintenance programs, we offer various levels of service contracts.
Due to the proximity of our locations to customers, we make sure that on-site technicians are available on short notice. The immediate first response is accessible through our customer service department. Our call center assists you by walking him through several steps on the phone.
SPARE AND WEAR TEAR
Our warehouses hold extensive inventories of spare and wear parts. Our ERP system enables us to locate spare parts and expedite them immediately.
If we do not have a particular part in stock, our relationship with reliable vendors enables us to supply you with the needed parts quickly. Non-purchased machine parts are marked and can be reproduced in our in-house machine shops at any time, ensuring 100% inspected high-quality parts.
 Focus on patient safety: fast and accurate checking of packaged medicines
Focus on patient safety: fast and accurate checking of packaged medicines
For pharmaceutical, cosmetics, and other healthcare companies, the use of check weighing systems increase the efficiency of making the product completeness check downstream of the packaging process. This starts with a quick check at blister pack level to verify that a blister pack is missing. This process also applies to quality assurance, where our highly accurate check weighers detect whether every folding box includes an information leaflet.
Our checkweighers accurately detect errors by enabling product measurements in the milligram range. Another strong argument in favor of our checkweighing systems is the fact that we eject faulty products after verification and separate them according to attributes. All this takes place dynamically and while your process continues to run unaffected.
 In-process control: Boost your efficiency with in-process inspections
Boost the reliability and efficiency of your production process by conducting quality control checks before the final inspection takes place. Our integrable inspection systems allow you to detect defects long before they cause any damage.
In-process control: Boost your efficiency with in-process inspections
Boost the reliability and efficiency of your production process by conducting quality control checks before the final inspection takes place. Our integrable inspection systems allow you to detect defects long before they cause any damage. We design our isolators to guarantee aseptic conditions inside the machine processing area and to protect operators from product toxicity. These isolators, which guarantee sterility and containment, are deployed as filling equipment for the production of injectable drugs needed for oncologic treatments.
We design our isolators to guarantee aseptic conditions inside the machine processing area and to protect operators from product toxicity. These isolators, which guarantee sterility and containment, are deployed as filling equipment for the production of injectable drugs needed for oncologic treatments.
Our isolator, based on quality and experience, allows us to provide customers with modern equipment, fully integrated with our filling lines and compliant to the latest regulation, such as EU and FDA cGMP, CE and UL.
All our isolators are designed and manufactured with the following features:
Possibility of installation of an independent safety barrier on each single glove.
The combination of Isolator & filling machine satisfies the containment level Class 3, ISO 10648-02
We not only deliver state-of-the-art packaging machines but also ensure comprehensive line integration for the entire range of your production requirements.
Thanks to our experts’ well-founded expertise and our cooperative partnership with you, our customers, we find new ways to realize challenging projects. We offer you our comprehensive product portfolio, consisting of consulting, software, machines, and packaging materials, so that we can provide you with tailored packaging solutions.
Training specific programs are used during FAT operations and/or during installation of the lines and/or single machines. This is to allow an easy and quick personnel training package for the machine use. We will give instruction on the machine operation, care, maintenance, size change-over procedures, calibration and set-up.
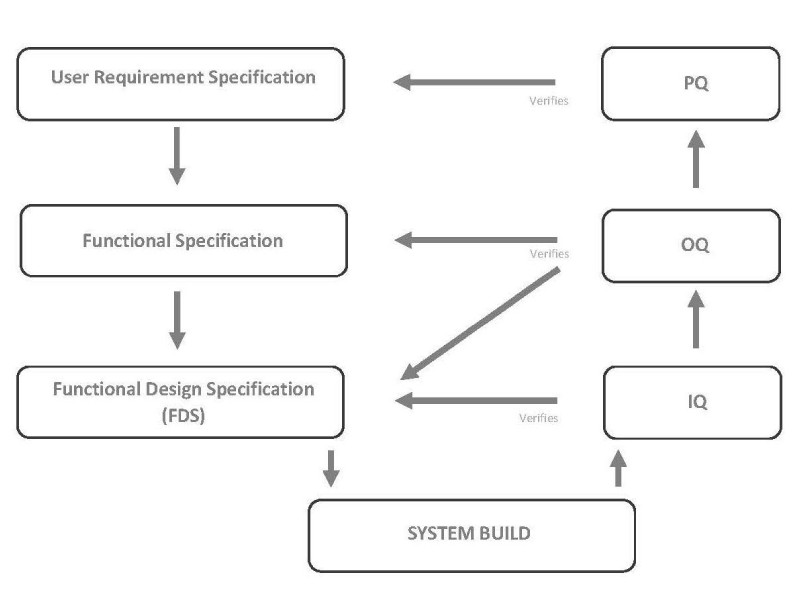 Validation Protocol is focused on customer’s total satisfaction, reason why preparation of Validation Protocol in accordance with cGMP and GAMP standards is one of the main activity of our engineer’s team.
Validation Protocol is focused on customer’s total satisfaction, reason why preparation of Validation Protocol in accordance with cGMP and GAMP standards is one of the main activity of our engineer’s team.
The purpose of validation is to establish documented evidence providing a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications (requirements) and quality attributes.
The main stages are indicated below:
Dwarkesh Pharma supplies FAT (Factory Acceptance Test) protocol validation and SAT (Site Acceptance Test) protocol; on request IQ & OQ protocols (Installation Qualification & Operational Qualification).
The complete validation test for the equipment will be performed at MAR’s site, following complete test list included in the FAT Protocol.
Dwarkesh Pharma can preform if requested, as an optional service, SAT Protocol or IQ-OQ validation Protocol at customer’s site.

Constant advances in technology require companies to upgrade and/or retrofit their current equipment to meet the latest industry standards.
Retrofitting offers the opportunity to modify and upgrade an old machine in such a way that it can be used in production for many more years. It has the advantage of providing a fully functioning machine that meets the current standards without having to make a major investment in new equipment. can analyze your existing machines and recommend solutions to repair, upgrade, optimize, and re-introduce an older machine into a production line. The retrofitting may involve mechanical and electrical, pneumatic, or software upgrades, depending on the machine’s condition.
Our core competence in project management delivers results. Upon acknowledgment of an order, a dedicated Project Manager will be your contact throughout each project phase.